The molar mass and molecular weight of NH 4 (3PO 4) is 302.952. Convert NH4 (3PO4) From Moles to Grams Moles mol Convert NH4 (3PO4) From Grams to Moles and Molecules Weight g Composition of NH 4 (3PO 4)
Answered: What is the mass of solute in each of… | bartleby
Molar Mass, Molecular Weight and Elemental Composition Calculator. Molar mass of ( (NH4)3PO4) is 149.0867 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between ( (NH4)3PO4) weight and moles. Compound.

Source Image: scribd.com
Download Image
29K views 2 years ago Explanation of how to find the molar mass of (NH4)3PO4: Ammonium phosphate. Note that diammonium phosphate (NH4)2HPO4 and monoammonium salt (NH4)H2PO4 ar …more

Source Image: youtube.com
Download Image
SOLVED: How many moles of NH4+ ions are there in 0.75g of (NH4)3PO4? Question 2 options: 9.09 x 1021 mol 0.0151 mol 0.00503 mol 3.03 x 1021 mol Chemistry Chemistry questions and answers 0.75 moles of (NH4)3PO4 = _________________ g This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: 0.75 moles of (NH4)3PO4 = _________________ g 0.75 moles of (NH4)3PO4 = _________________ g

Source Image: scribd.com
Download Image
What Is The Mass Of 0.75 Moles Of Nh4 3po4
Chemistry Chemistry questions and answers 0.75 moles of (NH4)3PO4 = _________________ g This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: 0.75 moles of (NH4)3PO4 = _________________ g 0.75 moles of (NH4)3PO4 = _________________ g Powered by AnyClip College May Be Less Expensive Than You Think More information from the unit converter How many moles (NH4)3PO4 in 1 grams? The answer is 0.0067075045929135. We assume you are converting between moles (NH4)3PO4 and gram .
Books Doubtnut Question Bank PDF | PDF | Mole (Unit) | Gases
There are 4 easy steps to find the molar mass of (NH4)3PO4 based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of Tribasic Ammonium Phosphate is to count the number of each atom present in a single molecule using the chemical formula, (NH4)3PO4: 2. Find Atomic Mass of Each Element. Stoichiometry. – ppt download

Source Image: slideplayer.com
Download Image
Basic Chemical Calculations-Merged | PDF | Mole (Unit) | Chemical Elements There are 4 easy steps to find the molar mass of (NH4)3PO4 based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of Tribasic Ammonium Phosphate is to count the number of each atom present in a single molecule using the chemical formula, (NH4)3PO4: 2. Find Atomic Mass of Each Element.

Source Image: scribd.com
Download Image
Answered: What is the mass of solute in each of… | bartleby The molar mass and molecular weight of NH 4 (3PO 4) is 302.952. Convert NH4 (3PO4) From Moles to Grams Moles mol Convert NH4 (3PO4) From Grams to Moles and Molecules Weight g Composition of NH 4 (3PO 4)
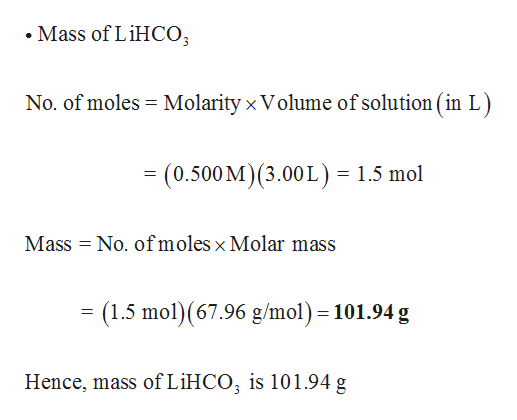
Source Image: bartleby.com
Download Image
SOLVED: How many moles of NH4+ ions are there in 0.75g of (NH4)3PO4? Question 2 options: 9.09 x 1021 mol 0.0151 mol 0.00503 mol 3.03 x 1021 mol 29K views 2 years ago Explanation of how to find the molar mass of (NH4)3PO4: Ammonium phosphate. Note that diammonium phosphate (NH4)2HPO4 and monoammonium salt (NH4)H2PO4 ar …more

Source Image: numerade.com
Download Image
Calculate the molar mass of each of the following: (a) (NH4) | Quizlet Compound Moles Weight, g (NH 4) 3 PO 4 Elemental composition of (NH4)3PO4 Chemical structure Appearance Ammonium phosphate appears as a white crystalline solid. Sample reactions for (NH4)3PO4 Related compounds Formula in Hill system is H12N3O4P Computing molar mass (molar weight)
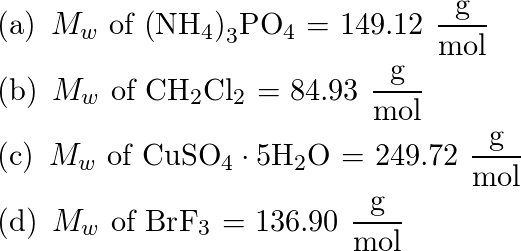
Source Image: quizlet.com
Download Image
what is the molar mass of (nh4)3po4 | Quizlet Chemistry Chemistry questions and answers 0.75 moles of (NH4)3PO4 = _________________ g This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: 0.75 moles of (NH4)3PO4 = _________________ g 0.75 moles of (NH4)3PO4 = _________________ g

Source Image: quizlet.com
Download Image
Determine the mass, in grams, of 0.0025 moles of ammonium phosphate – YouTube Powered by AnyClip College May Be Less Expensive Than You Think More information from the unit converter How many moles (NH4)3PO4 in 1 grams? The answer is 0.0067075045929135. We assume you are converting between moles (NH4)3PO4 and gram .

Source Image: youtube.com
Download Image
Basic Chemical Calculations-Merged | PDF | Mole (Unit) | Chemical Elements
Determine the mass, in grams, of 0.0025 moles of ammonium phosphate – YouTube Molar Mass, Molecular Weight and Elemental Composition Calculator. Molar mass of ( (NH4)3PO4) is 149.0867 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between ( (NH4)3PO4) weight and moles. Compound.
SOLVED: How many moles of NH4+ ions are there in 0.75g of (NH4)3PO4? Question 2 options: 9.09 x 1021 mol 0.0151 mol 0.00503 mol 3.03 x 1021 mol what is the molar mass of (nh4)3po4 | Quizlet Compound Moles Weight, g (NH 4) 3 PO 4 Elemental composition of (NH4)3PO4 Chemical structure Appearance Ammonium phosphate appears as a white crystalline solid. Sample reactions for (NH4)3PO4 Related compounds Formula in Hill system is H12N3O4P Computing molar mass (molar weight)