Pour the citric acid solution into a coffee cup. Use a thermometer or other temperature probe to record the initial temperature. Stir in the baking soda — sodium bicarbonate. Track the change in temperature as a function of time. The reaction is: H 3 C 6 H 5 O 7 (aq) + 3 NaHCO 3 (s) → 3 CO 2 (g) + 3 H 2 O (l) + Na 3 C 6 H 5 O 7 (aq)
Recipes for Natural Living – This is a pic of the ingred list on the back of our Buzz Away bottle. | Facebook
Jul 27, 2023In the presence of water, citric acid and sodium bicarbonate (aka baking soda) react to form sodium citrate, water, and carbon dioxide. Students investigate this endothermic reaction.
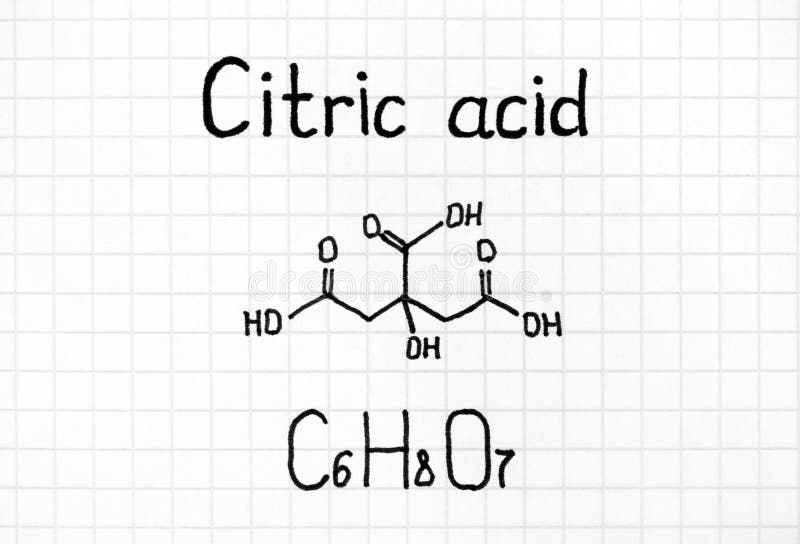
Source Image: dreamstime.com
Download Image
Based on their experience with the activities in this lesson, students are able to explain that carbon dioxide gas produced in the chemical reaction between citric acid and baking soda causes these balloons to inflate.
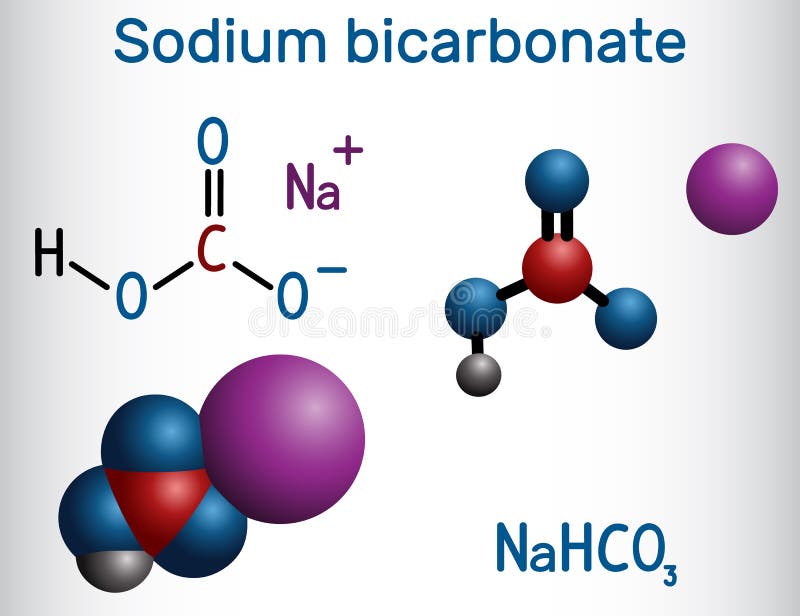
Source Image: dreamstime.com
Download Image
warmup – endothermic or exothermic?? – ppt download AlkaSeltzer contains sodium bicarbonate (NaHCO3) and citric acid (H3C6H5O7). When a tablet is dissolved in water, the following reaction produces sodium citrate, water, and carbon dioxide (gas): Step-by-Step Solution Given Information

Source Image: slideplayer.com
Download Image
Sodium Bicarbonate And Citric Acid Balanced Equation
AlkaSeltzer contains sodium bicarbonate (NaHCO3) and citric acid (H3C6H5O7). When a tablet is dissolved in water, the following reaction produces sodium citrate, water, and carbon dioxide (gas): Step-by-Step Solution Given Information Looking at the third trial, they have 0.41 grams of sodium bicarbonate, and 0.30 grams of citric acid. Using the molar masses of NaHCO 3 and C 6 H 8 O 7, they can calculate that there are 0.0049 moles and 0.0016 moles respectively. This is a 3:1 ratio. To put all the pieces together, one more bit of information is needed — the balanced equation!
Limiting and Excess Lab How much do I have left over? How much can be produced? – ppt download
HSPS1-2: Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties. How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) – YouTube

Source Image: m.youtube.com
Download Image
All Things Being Eco – Citric Acid Powder | Zero Waste Bulk Ingredient HSPS1-2: Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.

Source Image: allthingsbeingeco.ca
Download Image
Recipes for Natural Living – This is a pic of the ingred list on the back of our Buzz Away bottle. | Facebook Pour the citric acid solution into a coffee cup. Use a thermometer or other temperature probe to record the initial temperature. Stir in the baking soda — sodium bicarbonate. Track the change in temperature as a function of time. The reaction is: H 3 C 6 H 5 O 7 (aq) + 3 NaHCO 3 (s) → 3 CO 2 (g) + 3 H 2 O (l) + Na 3 C 6 H 5 O 7 (aq)

Source Image: m.facebook.com
Download Image
warmup – endothermic or exothermic?? – ppt download Based on their experience with the activities in this lesson, students are able to explain that carbon dioxide gas produced in the chemical reaction between citric acid and baking soda causes these balloons to inflate.

Source Image: slideplayer.com
Download Image
Food Grade Sodium Bicarbonate / Tech Grade Sodium Bicarbonate – China Baking Soda, Soda | Made-in-China.com 1. Show that the equivalence amount of citric acid for 2.00 g of sodium bicarbonate is 1.52 g. 2. Calculate the theoretical yield of carbon dioxide from 1.01 g of sodium bicarbonate. 3. Calculate the percentage yield in the plastic cup A.

Source Image: longhongchem.en.made-in-china.com
Download Image
How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) – YouTube AlkaSeltzer contains sodium bicarbonate (NaHCO3) and citric acid (H3C6H5O7). When a tablet is dissolved in water, the following reaction produces sodium citrate, water, and carbon dioxide (gas): Step-by-Step Solution Given Information

Source Image: m.youtube.com
Download Image
How to Neutralize Citric Acid? | Shanghai Chemex Looking at the third trial, they have 0.41 grams of sodium bicarbonate, and 0.30 grams of citric acid. Using the molar masses of NaHCO 3 and C 6 H 8 O 7, they can calculate that there are 0.0049 moles and 0.0016 moles respectively. This is a 3:1 ratio. To put all the pieces together, one more bit of information is needed — the balanced equation!
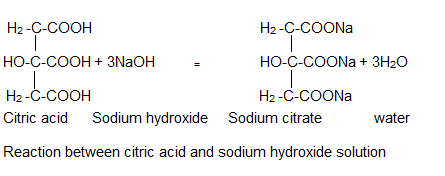
Source Image: shanghaichemex.com
Download Image
All Things Being Eco – Citric Acid Powder | Zero Waste Bulk Ingredient
How to Neutralize Citric Acid? | Shanghai Chemex Jul 27, 2023In the presence of water, citric acid and sodium bicarbonate (aka baking soda) react to form sodium citrate, water, and carbon dioxide. Students investigate this endothermic reaction.
warmup – endothermic or exothermic?? – ppt download How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) – YouTube 1. Show that the equivalence amount of citric acid for 2.00 g of sodium bicarbonate is 1.52 g. 2. Calculate the theoretical yield of carbon dioxide from 1.01 g of sodium bicarbonate. 3. Calculate the percentage yield in the plastic cup A.